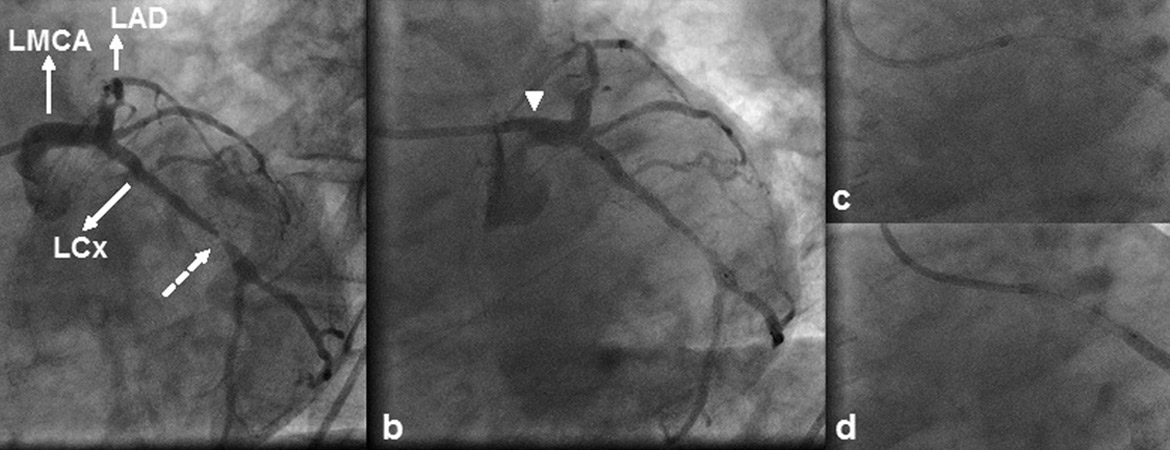
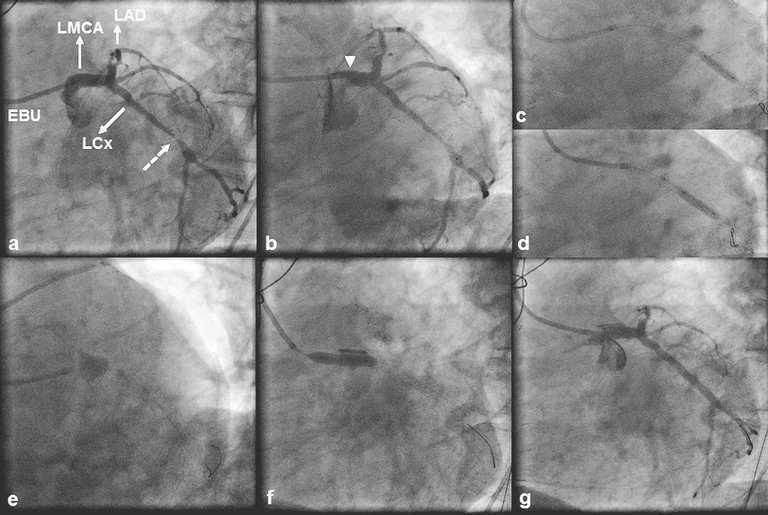
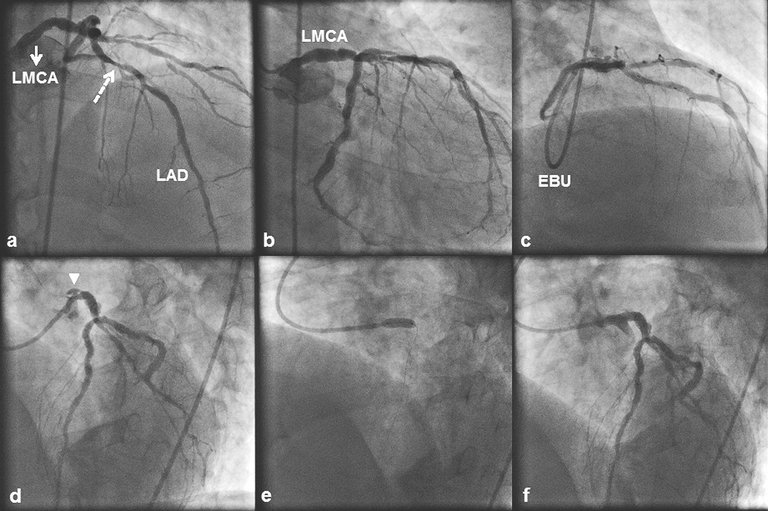
1 Lee SW, Hong MK, Kim YH, Park JH, Rhee KS, Lee CW, et al. Bail-out stenting for left main coronary artery dissection during catheter-based procedure: acute and long-term results.Clin Cardiol. 2004;27(7):393–5.
2 Cheng CI, Wu CJ, Hsieh YK, Chen YH, Chen CJ, Chen SM, et al. Percutaneous coronary intervention for iatrogenic left main coronary artery dissection. Int J Cardiol. 2008;126(2):177–82.
3 Onsea K, Kayaert P, Desmet W, Dubois CL. Iatrogenic left main coronary artery dissection. Neth Heart J 2011;19(4):192–5.
4 Eshtehardi P, Adorjan P, Togni M, Tevaearai H, Vogel R, Seiler C,
et al. Iatrogenic left main coronary artery dissection: incidence, classification, management, and long-term follow-up. Am Heart J 2010;159(6):1147–53.
5 Rogers JH, Lasala JM. Coronary artery dissection and perforation complicating percutaneous coronary intervention. J Invasive Cardiol.2004;16(9):493–9.
6 Huber MS, Mooney JF, Madison J, Mooney MR. Use of a morphologic classification to predict clinical outcome after dissection from coronary angioplasty. Am J Cardiol.1991;68(5):467–71.
7 Klein LW. Coronary complications of percutaneous coronary intervention: a practical approach to the management of abrupt closure. Catheter Cardiovasc Interv. 2005;64(3):395–401.
8 de Bono D. Complications of diagnostic cardiac catheterisation: results from 34 041 patients in the United Kingdom confidential enquiry into cardiac catheter complications. The Joint Audit Committee of the British Cardiac Society and Royal College of Physicians of London.Br Heart J.1993;70(3):297–300.
9 Holmes DR Jr, Holubkov R, Vlietstra RE, Kelsey SF, Reeder GS, Dorros G, et al. Comparison of complications during percutaneous transluminal coronary angioplasty from 1977 to 1981 and from 1985 to 1986: the National Heart, Lung, and Blood Institute Percutaneous Transluminal Coronary Angioplasty Registry. J Am Coll Cardiol. 1988;12(5):1149–55.
10 West R, Ellis G, Brooks N. Complications of diagnostic cardiac catheterisation: results from a confidential inquiry into cardiac catheter complications. Heart. 2006;92(6):810–4.
11 Mattichak SJ, Dixon SR, Shannon F, Boura JA, Safian RD. Failed percutaneous coronary intervention: a decade of experience in 21 000 patients. Catheter Cardiovasc Interv.2008;71(2):131–7.
12 Boyle AJ, Chan M, Dib J, Resar J. Catheter-induced coronary artery dissection: risk factors, prevention and management. J Invasive Cardiol.2006;18(10):500–3.
13 Ammann P, Brunner-La Rocca HP, Angehrn W, Roelli H, Sagmeister M, Rickli H. Procedural complications following diagnostic coronary angiography are related to the operator’s experience and the catheter size. Catheter Cardiovasc Interv.2003;59(1):13–8.
14 Hibbert B, Simard T, Wilson KR, Hawken S, Wells GA, Ramirez FD,
et al. Transradial versus transfemoral artery approach for coronary angiography and percutaneous coronary intervention in the extremely obese. JACC Cardiovasc Interv.2012;5(8):819–26.
15 Caputo RP, Tremmel JA, Rao S, Gilchrist IC, Pyne C, Pancholy S, et al. Transradial arterial access for coronary and peripheral procedures: executive summary by the Transradial Committee of the SCAI. Catheter Cardiovasc Interv. 2011;78(6):823–39.
16 Zidi M, Nallet O, Esteve JB, Michaud P, Cattan S. Extensive iatrogenic coronary dissection during coronary angioplasty: a series
of 19 consecutive patients. Ann Cardiol Angeiol (Paris). 2010;59(5):306–10.
17 López-Mínguez JR, Climent V, Yen-Ho S, González-Fernández R, Nogales-Asensio JM, Sánchez-Quintana D. Structural features of the sinus of valsalva and the proximal portion of the coronary arteries: their relevance to retrograde aortocoronary dissection. Rev Esp Cardiol.2006;59(7):696–702.
18 Gómez-Moreno S, Sabaté M, Jiménez-Quevedo P, Vázquez P, Alfonso F, Angiolillo DJ, et al. Iatrogenic dissection of the ascending aorta following heart catheterisation: incidence, management and outcome. EuroIntervention.2006;2(2):197–202.
19 Núñez-Gil IJ, Bautista D, Cerrato E, Salinas P, Varbella F, Omedè P, et al. Incidence, Management, and Immediate- and Long-Term Outcomes After Iatrogenic Aortic Dissection During Diagnostic or Interventional Coronary Procedures. Circulation 2015;131(24):2114–9.
20 Pavei A, Marco J. Tools & techniques: choice and use of guiding catheters. EuroIntervention.2010;6(4):543–4.
21 Dunning DW, Kahn JK, Hawkins ET, O’Neill WW. Iatrogenic coronary artery dissections extending into and involving the aortic root. Catheter Cardiovasc Interv.2000;51(4):387–93.
22 Murat C, Uygar CY, Emre Y, Yalcin G, Atila I. Conservative treatment of iatrogenic left main coronary artery dissection: report of two cases. Cardiovasc Diagn Ther. 2013;3(4):244–6.
23 Zelinger AB, Shulruff S, Pouget JM. Significant left main stenosis following asymptomatic dissection during coronary arteriography. Chest 1983;83(3):568–9.
24 Sanidas E, Buysschaert I, van Langenhove G. Iatrogenic left main coronary artery dissection and intramural hematoma caused by diagnostic transradial cardiac catheterization. Hellenic J
Cardiol.2014;55(1):65–9.
25 Binder RK, Boone RH, Webb JG. Left main dissection conservatively managed with optical coherence tomography guidance. Catheter Cardiovasc Interv.2014;83(1):65–8.