1 Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al.; Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. doi:
https://doi.org/10.1093/eurheartj/ehw128.
2 Braunwald E. Heart failure. JACC Heart Fail. 2013;1(1):1–20. doi:
https://doi.org/10.1016/j.jchf.2012.10.002.
3 Rudiger A, Harjola V-P, Müller A, Mattila E, Säila P, Nieminen M, et al. Acute heart failure: clinical presentation, one-year mortality and prognostic factors. Eur J Heart Fail. 2005;7(4):662–70. doi:
https://doi.org/10.1016/j.ejheart.2005.01.014.
4 Arrigo M, Gayat E, Parenica J, Ishihara S, Zhang J, Choi DJ, et al.; GREAT Network. Precipitating factors and 90-day outcome of acute heart failure: a report from the intercontinental GREAT registry. Eur J Heart Fail. 2017;19(2):201–8. doi:
https://doi.org/10.1002/ejhf.682.
5 Arrigo M, Parissis JT, Akiyama E, Mebazaa A. Understanding acute heart failure: pathophysiology and diagnosis. Eur Heart J Suppl. 2016;18(suppl G):G11–8. doi:
https://doi.org/10.1093/eurheartj/suw044.
6 McKie PM, Schirger JA, Costello-Boerrigter LC, Benike SL, Harstad LK, Bailey KR, et al. Impaired natriuretic and renal endocrine response to acute volume expansion in pre-clinical systolic and diastolic dysfunction. J Am Coll Cardiol. 2011;58(20):2095–103. doi:
https://doi.org/10.1016/j.jacc.2011.07.042.
7 Zile MR, Bennett TD, St John Sutton M, Cho YK, Adamson PB, Aaron MF, et al. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118(14):1433–41. doi:
https://doi.org/10.1161/CIRCULATIONAHA.108.783910.
8 Nijst P, Verbrugge FH, Grieten L, Dupont M, Steels P, Tang WH, et al. The pathophysiological role of interstitial sodium in heart failure. J Am Coll Cardiol. 2015;65(4):378–88. doi:
https://doi.org/10.1016/j.jacc.2014.11.025.
9 Cotter G, Metra M, Milo-Cotter O, Dittrich HC, Gheorghiade M. Fluid overload in acute heart failure – re-distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail. 2008;10(2):165–9. doi:
https://doi.org/10.1016/j.ejheart.2008.01.007.
10 Gandhi SK, Powers JC, Nomeir AM, Fowle K, Kitzman DW, Rankin KM, et al. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med. 2001;344(1):17–22. doi:
https://doi.org/10.1056/NEJM200101043440103.
11 Viau DM, Sala-Mercado JA, Spranger MD, O’Leary DS, Levy PD. The pathophysiology of hypertensive acute heart failure. Heart. 2015;101(23):1861–7. doi:h
ttps://doi.org/10.1136/heartjnl-2015-307461.
12 Colombo PC, Onat D, Harxhi A, Demmer RT, Hayashi Y, Jelic S, et al. Peripheral venous congestion causes inflammation, neurohormonal, and endothelial cell activation. Eur Heart J. 2014;35(7):448–54. doi:
https://doi.org/10.1093/eurheartj/eht456.
13 Harjola V-P, Mullens W, Banaszewski M, Bauersachs J, Brunner-La Rocca HP, Chioncel O, et al. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail. 2017;19(7):821–36. doi:
https://doi.org/10.1002/ejhf.872.
14 Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53(7):589–96. doi:
https://doi.org/10.1016/j.jacc.2008.05.068.
15 Poelzl G, Ess M, Mussner-Seeber C, Pachinger O, Frick M, Ulmer H. Liver dysfunction in chronic heart failure: prevalence, characteristics and prognostic significance. Eur J Clin Invest. 2012;42(2):153–63. doi:
https://doi.org/10.1111/j.1365-2362.2011.02573.x.
16 Ishihara S, Gayat E, Sato N, Arrigo M, Laribi S, Legrand M, et al. Similar hemodynamic decongestion with vasodilators and inotropes: systematic review, meta-analysis, and meta-regression of 35 studies on acute heart failure. Clin Res Cardiol. 2016;105(12):971–80. doi:
https://doi.org/10.1007/s00392-016-1009-6.
17 Mebazaa A, Yilmaz MB, Levy P, Ponikowski P, Peacock WF, Laribi S, et al. Recommendations on pre-hospital & early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine. Eur J Heart Fail. 2015;17(6):544–58. doi:
https://doi.org/10.1002/ejhf.289.
18 Forrester JS, Diamond G, Chatterjee K, Swan HJ. Medical therapy of acute myocardial infarction by application of hemodynamic subsets (first of two parts). N Engl J Med. 1976;295(24):1356–62. doi:
https://doi.org/10.1056/NEJM197612092952406.
19 Forrester JS, Diamond GA, Swan HJ. Correlative classification of clinical and hemodynamic function after acute myocardial infarction. Am J Cardiol. 1977;39(2):137–45. doi:
https://doi.org/10.1016/S0002-9149(77)80182-3.
20 Harjola V-P, Lassus J, Sionis A, Køber L, Tarvasmäki T, Spinar J, et al.; CardShock Study Investigators; GREAT network. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur J Heart Fail. 2015;17(5):501–9. doi:
https://doi.org/10.1002/ejhf.260.
21 Rudiger A, Gasser S, Fischler M, Hornemann T, von Eckardstein A, Maggiorini M. Comparable increase of B-type natriuretic peptide and amino-terminal pro-B-type natriuretic peptide levels in patients with severe sepsis, septic shock, and acute heart failure. Crit Care Med. 2006;34(8):2140–4. doi:
https://doi.org/10.1097/01.CCM.0000229144.97624.90.
22 Mebazaa A, Tolppanen H, Mueller C, Lassus J, DiSomma S, Baksyte G, et al. Acute heart failure and cardiogenic shock: a multidisciplinary practical guidance. Intensive Care Med. 2016;42(2):147–63. doi:
https://doi.org/10.1007/s00134-015-4041-5.
23 Cotter G, Metzkor E, Kaluski E, Faigenberg Z, Miller R, Simovitz A, et al. Randomised trial of high-dose isosorbide dinitrate plus low-dose furosemide versus high-dose furosemide plus low-dose isosorbide dinitrate in severe pulmonary oedema. Lancet. 1998;351(9100):389–93. doi:
https://doi.org/10.1016/S0140-6736(97)08417-1.
24 Matsue Y, Damman K, Voors AA, Kagiyama N, Yamaguchi T, Kuroda S, et al. Time-to-Furosemide Treatment and Mortality in Patients Hospitalized With Acute Heart Failure. J Am Coll Cardiol. 2017;69(25):3042–51. doi:
https://doi.org/10.1016/j.jacc.2017.04.042.
25 Mebazaa A, Parissis J, Porcher R, Gayat E, Nikolaou M, Boas FV, et al. Short-term survival by treatment among patients hospitalized with acute heart failure: the global ALARM-HF registry using propensity scoring methods. Intensive Care Med. 2011;37(2):290–301. doi:
https://doi.org/10.1007/s00134-010-2073-4.
26 Wilhelm MJ. Extracorporeal membrane oxygen ation for acute cardiogenic shock. Cardiovasc Med. 2016;19(2):39–43. doi:
https://doi.org/10.4414/cvm.2016.00393.
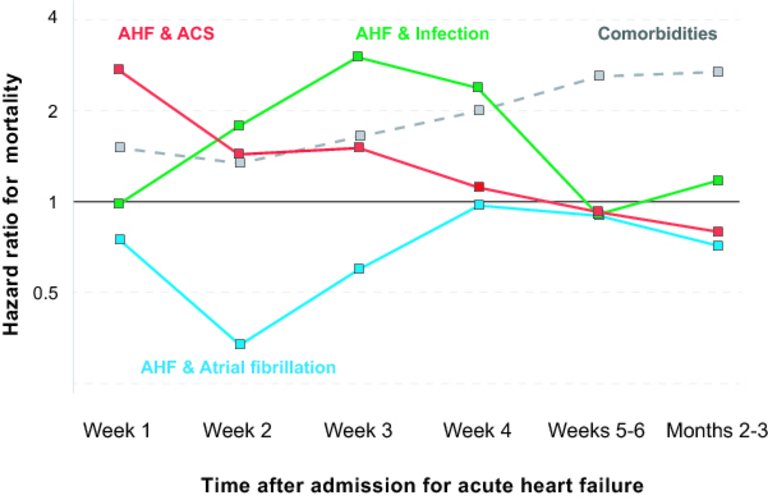
27 Arrigo M, Tolppanen H, Sadoune M, Feliot E, Teixeira A, Laribi S, et al.; GREAT Network. Effect of precipitating factors of acute heart failure on readmission and long-term mortality. ESC Heart Fail. 2016;3(2):115–21. doi:
https://doi.org/10.1002/ehf2.12083.
28 Rudiger A, Streit M, Businger F, Schmid ER, Follath F, Maggiorini M. The impact of infections on critically ill acute heart failure patients: an observational study. Swiss Med Wkly. 2010;140:w13125.
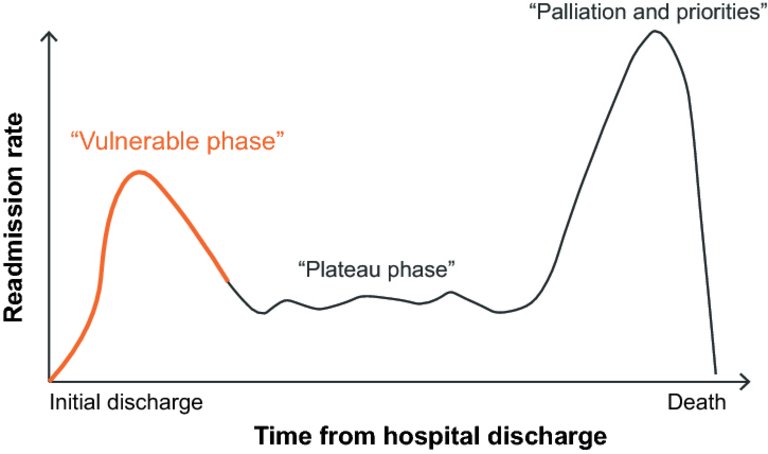
29 Greene SJ, Fonarow GC, Vaduganathan M, Khan SS, Butler J, Gheorghiade M. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol. 2015;12(4):220–9. doi:
https://doi.org/10.1038/nrcardio.2015.14.
30 Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63(12):1123–33. doi:
https://doi.org/10.1016/j.jacc.2013.11.053.
31 Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, et al.; EVEREST Trial Investigators. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34(11):835–43. doi:
https://doi.org/10.1093/eurheartj/ehs444.
32 Logeart D, Thabut G, Jourdain P, Chavelas C, Beyne P, Beauvais F, et al. Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol. 2004;43(4):635–41. doi:
https://doi.org/10.1016/j.jacc.2003.09.044.
33 Arrigo M, Truong QA, Onat D, Szymonifka J, Gayat E, Tolppanen H, et al. Soluble CD146 Is a Novel Marker of Systemic Congestion in Heart Failure Patients: An Experimental Mechanistic and Transcardiac Clinical Study. Clin Chem. 2017;63(1):386–93. doi:
https://doi.org/10.1373/clinchem.2016.260471.
34 Kubena P, Arrigo M, Parenica J, Gayat E, Sadoune M, Ganovska E, et al.; GREAT Network. Plasma Levels of Soluble CD146 Reflect the Severity of Pulmonary Congestion Better Than Brain Natriuretic Peptide in Acute Coronary Syndrome. Ann Lab Med. 2016;36(4):300–5. doi:
https://doi.org/10.3343/alm.2016.36.4.300.
35 Prins KW, Neill JM, Tyler JO, Eckman PM, Duval S. Effects of Beta-Blocker Withdrawal in Acute Decompensated Heart Failure: A Systematic Review and Meta-Analysis. JACC Heart Fail. 2015;3(8):647–53. doi:
https://doi.org/10.1016/j.jchf.2015.03.008.
36 Gayat E, Arrigo M, Littnerova S, Sato N, Parenica J, Ishihara S, et al.; GREAT Network. Heart failure oral therapies at discharge are associated with better outcome in acute heart failure: a propensity-score matched study. Eur J Heart Fail. 2017;18:613.
37 Komajda M, Tavazzi L, Swedberg K, Böhm M, Borer JS, Moyne A, et al.; SHIFT Investigators. Chronic exposure to ivabradine reduces readmissions in the vulnerable phase after hospitalization for worsening systolic heart failure: a post-hoc analysis of SHIFT. Eur J Heart Fail. 2016;18(9):1182–9. doi:
https://doi.org/10.1002/ejhf.582.
38 Hindricks G, Taborsky M, Glikson M, Heinrich U, Schumacher B, Katz A, et al.; IN-TIME study group*. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet. 2014;384(9943):583–90. doi:
https://doi.org/10.1016/S0140-6736(14)61176-4.
39 Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, et al.; CHAMPION Trial Study Group. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377(9766):658–66. doi:
https://doi.org/10.1016/S0140-6736(11)60101-3.