1 Rehn L. On operating cardiac injuries and cardiac suturing. Arch Klein Chir. 1897;55:315.
2 Tuffier T. État actuel de la chirurgie intrathoracique. Trans Int Congr Med. 1913 7; Surgery 1914;2:249.
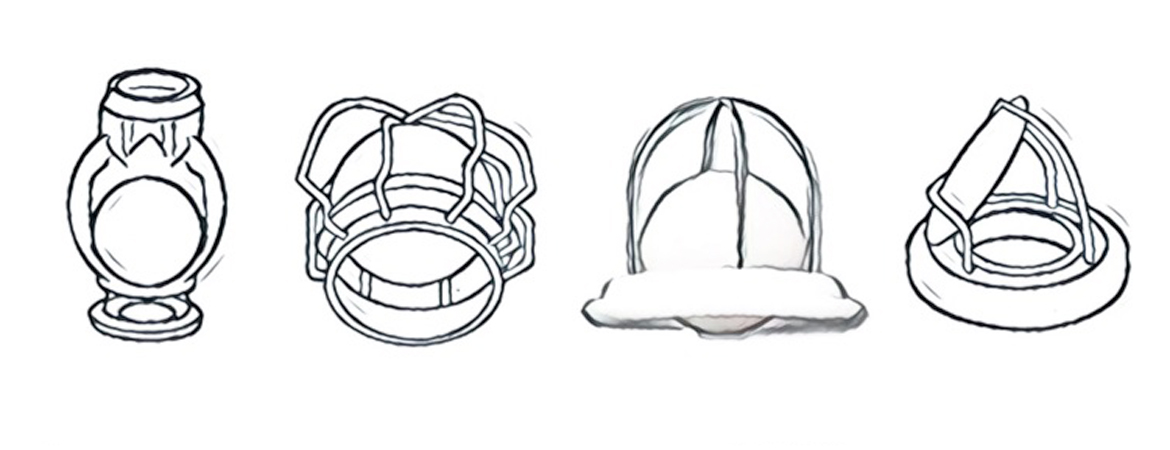
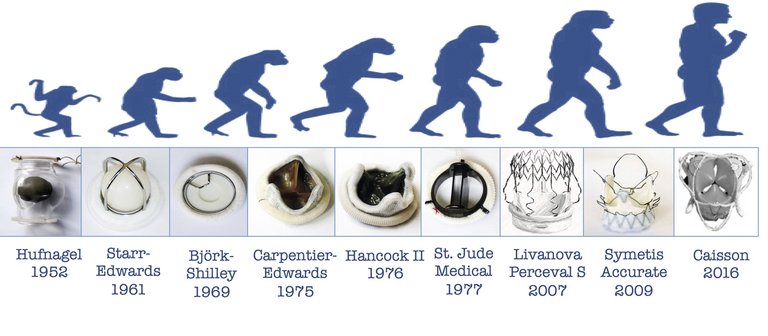
3 Hufnagel CA. Aortic plastic valvular prosthesis. Bull Georgetown Univ Med Center. 1951;5:128–30.
4 Von Segesser LK. The development of open valve surgery. In: Thoracic and Cardiovascular Surgery. From the magin mountain to rocket science. European Association for Cardio-Thoracic Sugery; 2010. p. 178–89.
5 Gott VL, Alejo DE, Cemeron DE. Mechanical heart valve: 50 years of evolutions. Ann Thorac Surg. 2003;76:S2230–9.
6 Chiariello L. Storia della Cardiochirurgia. In: Trattato di chirurgia Cardiaca. Universo Edition; 2016. p. 3–22.
7 Harken DE, Soro HS, Taylor WJ, et al. Partial and complete prostheses in aortic insufficiency. J Thorac Cardiovasc Surg. 1960;40:744–62.
8 Braunwald NS, Cooper T, Morrow AG. Clinical and experimental replacement of the mitral valve. Experience with the use of a flexible polyurethane prosthesis. in: Merendino K.A. (Ed.) Prosthetic Valves for Cardiac Surgery. Springfield, Ill.: Charles C Thomas; 1961. p. 307–31.
9 Starr A, Edwards ML. Mitral replacement: Clinical experience with a ball-valve prosthesis. Ann Surg. 1961;154:726–40.
10 Gödje OL1, Fischlein T, Adelhard K, Nollert G, Klinner W, Reichart B. Thirty-year results of Starr-Edwards prostheses in the aortic and mitral position. Ann Thorac Surg. 1997;63:613–9.
11 Gudbjartsson T, Absi T, Aranki S. Mitral valve replacement. In: Cohn L (ed.). Cardiac Surgery in the adult. New York: McGraw-Hill; p. 1032–68.
12 Chaikof EL. The Development of Prosthetic Heart Valves – Lessons in Form and Function. N Engl J Med. 2007;357:1368–71.
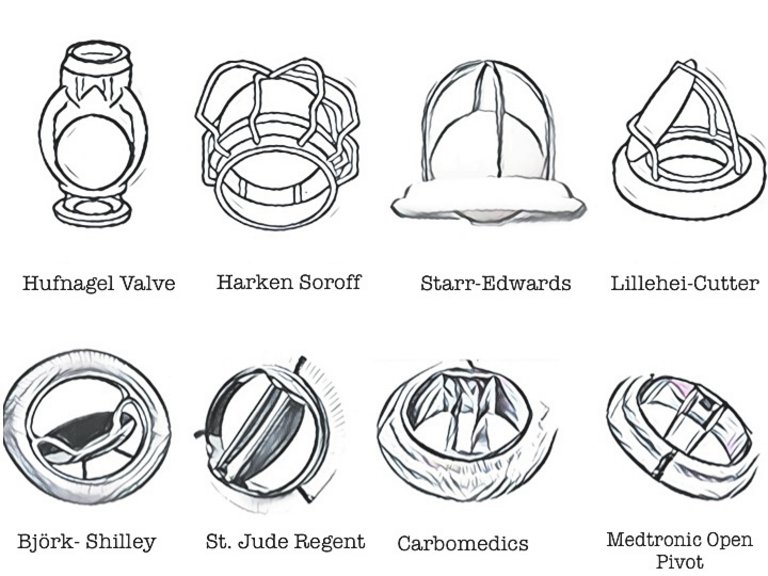
13 Lindblum D, Rodriguez L, Bjork VO. Mechanical failure of the Bjork-Shiley valve: updated follow-up and considerations on prophylactic replacement. J Thorac Cardiovasc Surg. 1989;97:95–7.
14 Nicoloff DM, Emery RW, Arom KV, et al. Clinical and hemodynamic results with the St. Jude Medical cardiac valve prosthesis. J Thorac Cardiovasc Surg. 1982;82:674–83.
15 Czer LS, Chaux A, Matloff JM, et al: Ten-year experience with the St Jude Medical valve for primary valve replacement. J Thorac Cardiovasc Surg. 1990;100:44; discussion 54.
16 Palatianos GM, Laczkovics AM, Simon P, et al. Multicentered European study on safety and effectiveness of the On-X prosthetic heart valve: intermediate follow-up. Ann Thorac Surg. 2007;83:40–6.
17 Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451–96.
18 Puskas J, Gerdisch M, Nichols D, et al. Reduced anticoagulation after mechanical aortic valve replacement: interim results from the prospective randomized on-X valve anticoagulation clinical trial randomized Food and Drug Administration investigational device exemption trial. J Thorac Cardiovasc Surg. 2014;147:1202–10.
19 Ross DN. Homograft replacement of the aortic valve. Lancet. 1962;2:487.
20 Ross DN. Evolution of the Homograft Valve. Ann Thorac Surg. 1995;59:565–7.
21 Spencer FC: Intellectual creativity in thoracic surgeons. J Thorac
Cardiovasc Surg. 1983;86:168.
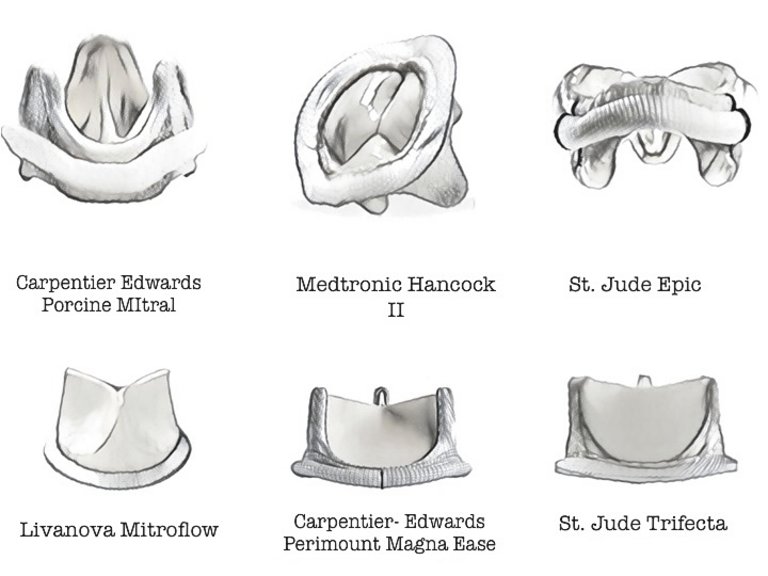
22 Carpetier A, Lemaigre G, Robert L, et al. Biological factors affecting long term results of valvular heterograft. Jour Thorac Card Vasc Surg. 1969;28:467–83.
23 Ioneuscu MI. The begining. In: The pericardial heart valve. Society for Cardiothoracic Surgery in Great Britain and Ireland; 2014; p. 1–35.
24 Rahimtoola SH. The problem of valve prosthesis-patient mismatch. Circulation. 1978;58:20–4.
25 Flameng W, Rega F, Vercalsteren M, et al. Antimineralization treatment and patient-prosthesis mismatch are major determinants of the onset and incidence of structural valve degeneration in bioprosthetic heart valves. J Thorac Cardiovasc Surg. 2014;147:1219–24.
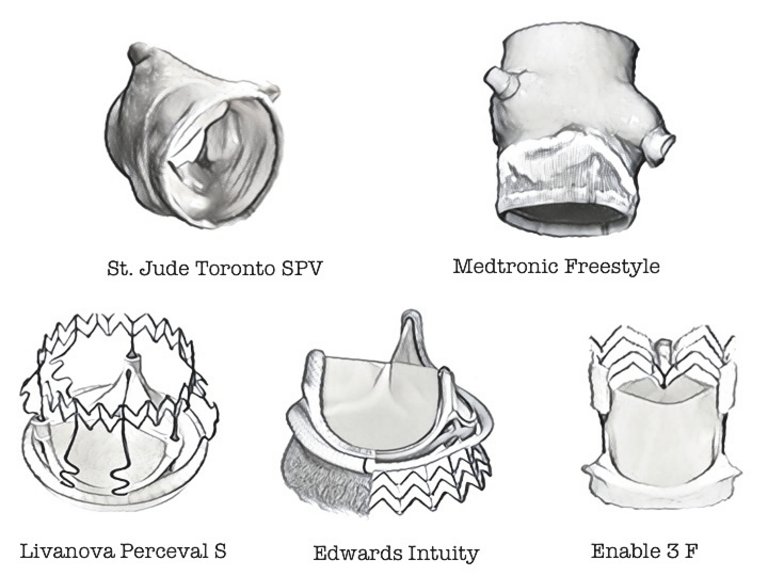
26 Desai ND, Merin O, Cohen GN, et al. Long-term results of aortic valve replacement with the St. Jude Toronto stentless porcine valve. Ann Thorac Surg. 2004;78:2076–83.
27 Cosgrove DM 3rd, Sabik JF. Minimally invasive approach for aortic valve operations. Ann Thorac Surg. 1996;62:596.
28 Osnabrugge RL, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62:1002–12.
29 Comendador JM, Castano M, Gualis J et al. Sutureless aortic bioprosthesis. Interact Card Vasc Surg. 2018:1–8.
30 Shrestha M, Fischlein T, Meuris B, et al. European multicentre experience with the sutureless perceval valve: clinical and haemodynamic outcomes up to 5 years in over 700 patients. Eur J Cardiothorac Surg. 2016;49:234–41.
31 Maisano F, Nietlispach F. The future of valve for percutanues insertion. Eur Heart J. 2014:35:1569–74.
32 Bonhoeffer P, Boudjemline Y, Saliba Z et al. Percutaneous replacement of pulmonary valve in a right- ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;21:9239.
33 Cribier A, Eltchaninoff H, Bash A et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation. 2002;106:3006–8.
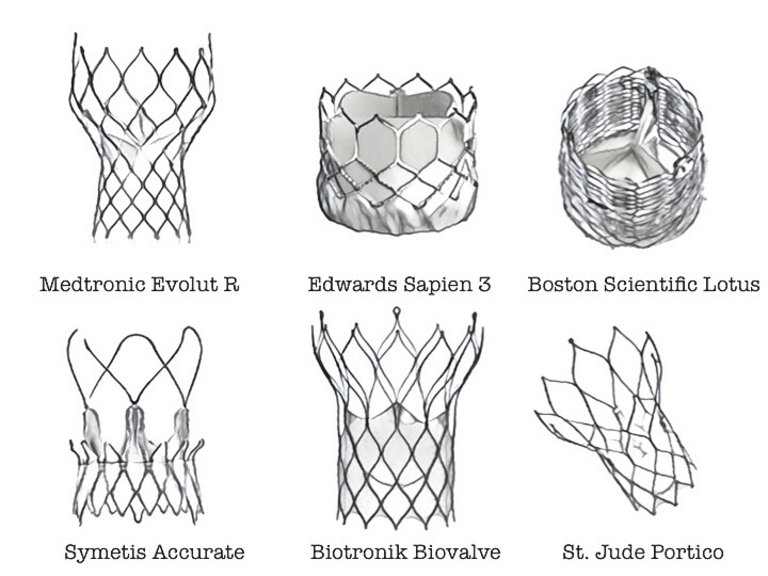
34 Abdel-Wahab M, Jose J, Richardt G. Transfemoral TAVI devices: design overview and clinical outcomes. EuroIntervention. 2015;11(Suppl W):W114–8.
35 Reardon MJ, Van Mieghem NM, Popma JJ. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2017;376:1321–31.
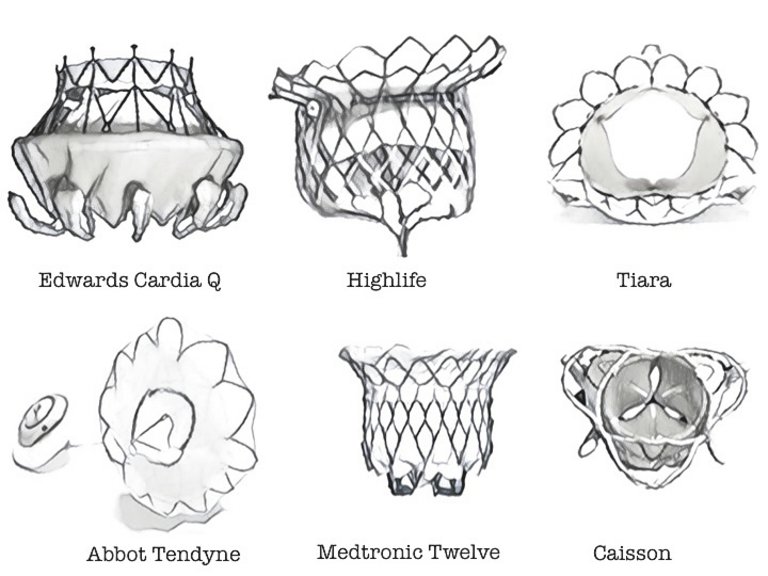
36 Maisano F, Kuwata S, Nietlispach, et al. Transcatheter mitral valve repair and replacement.J Cardiovasc Med (Hagerstown). 2017;17 (Suppl 1):e134-e140.
37 Taramasso M, Kuwata S, Rodriguez Cetina Biefer H, et al. Percutaneous mitral valve repair and replacement: complementary or competitive techniques? EuroIntervention. 2016;12(Y):Y97–Y101.
38 Flameng W, et al. A randomized assessment of an advanced tissue preservation technology in the juvenile sheep model. J Thorac Cardiovasc Surg. 2015;149:340–5.
39 Fioretta ES, Dijkmann PE, Emmert MY, et al. The future of heart valve replacement: recent developments and translational challenges fo heart valve tissue engineering. J Tissue Eng Regen Med. 2016 Sep 30. doi: 10.1002/term.2326. [Epub ahead of print]
40 Emmert MY, Fioretta ES, Hoerstrup SP. Translational Challenges in Cardiovascular Tissue Engineering. Cardiovasc Transl Res 2017. doi: 10.1007/s12265-017-9728-2.