EMH Schweizerischer Ärzteverlag AG
Farnsburgerstrasse 8
CH-4132 Muttenz
+41 (0)61 467 85 44
support@swisshealthweb.ch
www.swisshealthweb.ch
Published on 19.12.2018
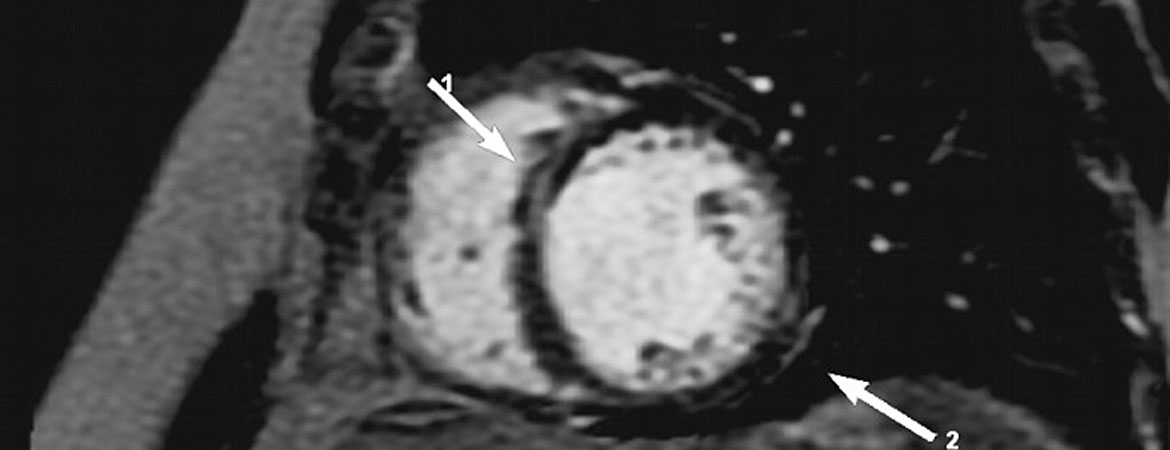
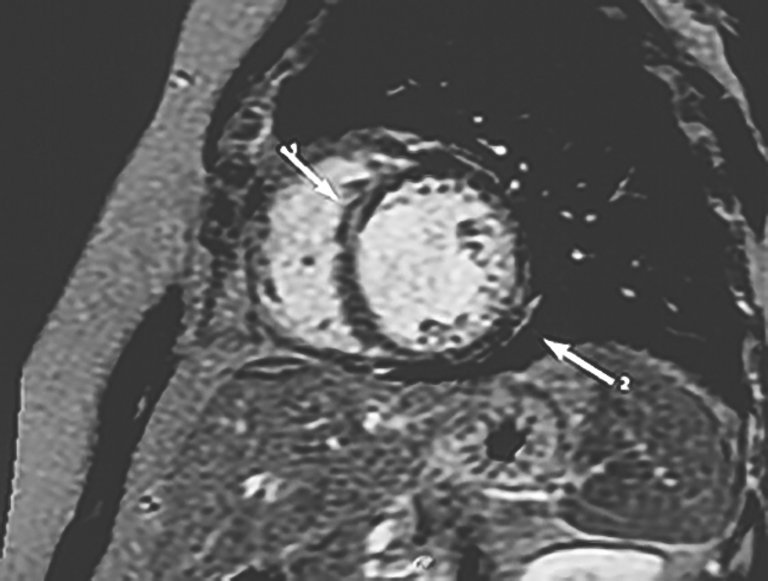
Chagas heart disease is becoming a worldwide health burden and represents a major cause of cardiovascular death in areas where it is endemic.
| Table 1: Patient’s laboratory test results. | ||
| Reference value | Patient’s value | |
| Haematology | ||
| Haemoglobin (g/l) | 120–160 | 145 |
| Mean corpuscular volume (fl) | 85–101 | 87 |
| Mean corpuscular haemoglobin (pg) | 28–33 | 30 |
| Mean corpuscular haemoglobin concentration (g/l) | 300–360 | 338 |
| Leucocyte count (G/l) | 3.5–10.0 | 5.8 |
| Platelet count (G/l) | 140–360 | 189 |
| Erythrocyte sedimation rate (mm/h) | <10 | 8 |
| Chemistry studies | ||
| Sodium (mmol/l) | 136–145 | 138 |
| Potassium (mmol/l) | 3.6–5.1 | 4.1 |
| Creatinine (µmol/l) | 49–90 | 70 |
| Urea (mmol/l) | 2.5–6.7 | 3.1 |
| C-reactive protein (mg/l) | <5.1 | 11.3+ |
| Troponin I high-sensitive (ng/l) | <26.3 | <10 |
| Creatinine kinase (U/l)) | 29–168 | 78 |
| Brain natriuretic peptide (ng/l) | <111 | 815+ |
| Alanine aminotransferase (U/l) | <35 | 44+ |
| Aspartate aminotransferase (U/l) | 5–31 | 33+ |
| Glycated haemoglobin (%) | <6% | 5.2 |
| Thyroid stimulating hormone (mU/l) | 0.35–4.94 | 11.60+ |
| Free tri-iodothyronine (fT3) (pmol/l) | 2.6–5.7 | 3.8 |
| Free thyroxine (fT4) (pmol/l) | 9–19 | 12 |
| Ferritin (μg/l) | 50–200 | 60 |
| Angiotensin converting enzyme (U/l) | 20–70 | 68 |
| Antinuclear antibodies (ANA) | <80 | <80 |
| p- ANCA (antineutrophil cytoplasmic antibodies) | <20 | <20 |
| c- ANCA | <20 | <20 |
| Serology | ||
| Anti-HBs (hepatitis B) | Negative | Negative |
| Anti-HBc (hepatitis B) | Negative | Negative |
| Anti-HCV (hepatitis C) | Negative | Negative |
| Anti-HIV 1 + 2 / p24-antigene | Negative | Negative |
| Anti-Treponema IgG | Negative | Negative |
| Anti-Treponema IgM | Negative | Negative |
| Anti-Trypanosoma (Chagas) ELISA | <0.30 | 1.66+ |
| Anti-Trypanosoma (Chagas) IFAT | <160 | 1280+ |
| ELISA = enzyme-linked immunosorbent assay; IFAT = indirect fluorescent antibody test; HIV = human immunodeficiency virus | ||
| Table 2: Rassi risk score for total long-term mortality in affected patients. | |
| Predictors of mortality | Points |
| New York Heart Association functional class III or IV | 5 |
| Cardiomegaly on chest radiography | 5 |
| Segmental or global wall motion abnormality | 3 |
| Non-sustained ventricular tachycardia on 24-hour ECG monitoring | 2 |
| Male sex | 2 |
| Maximum total | 17 |
Published under the copyright license.
"Attribution - Non-Commercial - NoDerivatives 4.0"
No commercial reuse without permission.
See: emh.ch/en/emh/rights-and-licences/