EMH Schweizerischer Ärzteverlag AG
Farnsburgerstrasse 8
CH-4132 Muttenz
+41 (0)61 467 85 44
support@swisshealthweb.ch
www.swisshealthweb.ch
Published on 12.03.2019
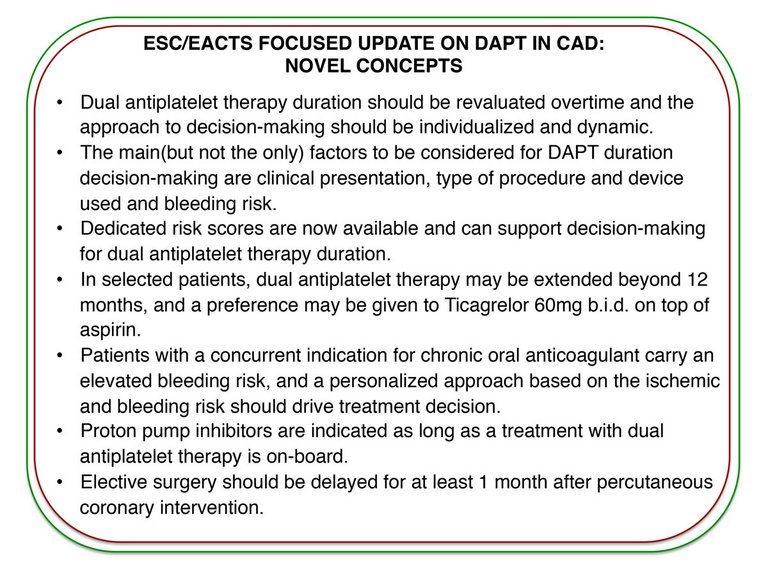
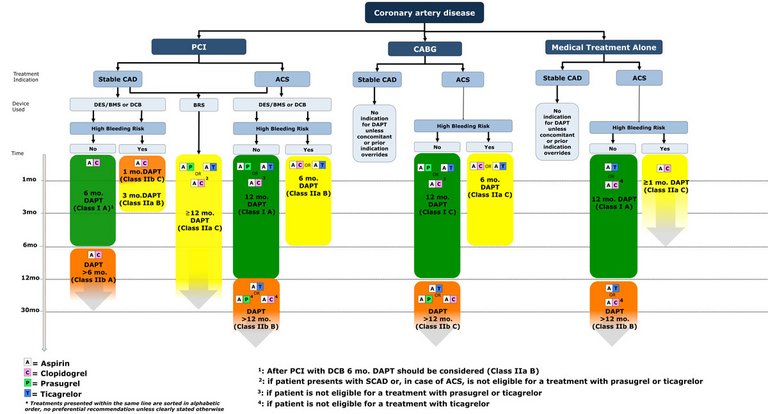
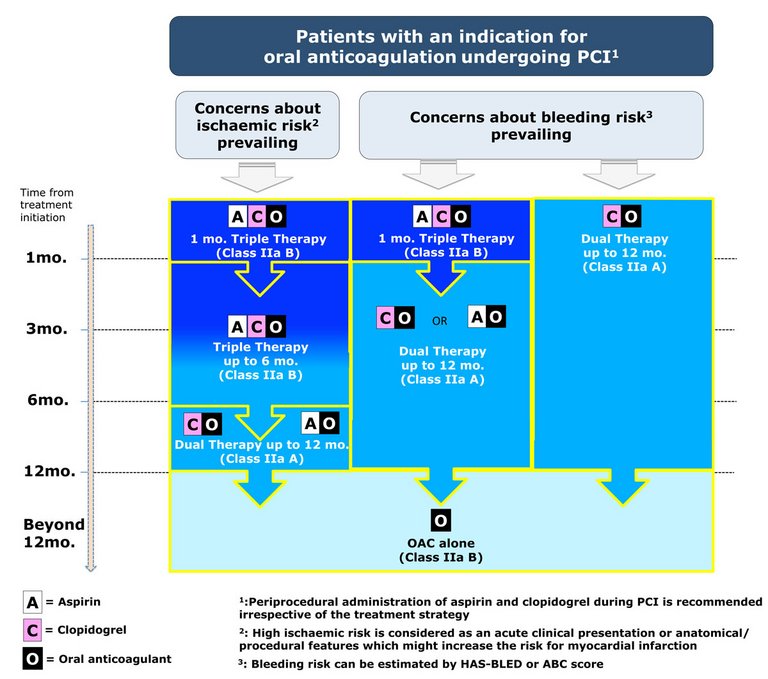
Dual antiplatelet therapy has been extensively studied in the last two decades, giving opportunities for thorough evidence-based recommendations. The ESC/EACTS guidelines on DAPT in CAD provide guidance for DAPT type and duration, maintaining a patient-centred focus. Patient-specific and dynamic evaluation of the ischaemic and bleeding risks is key for optimal treatment decisions.
Published under the copyright license.
"Attribution - Non-Commercial - NoDerivatives 4.0"
No commercial reuse without permission.
See: emh.ch/en/emh/rights-and-licences/