EMH Schweizerischer Ärzteverlag AG
Farnsburgerstrasse 8
CH-4132 Muttenz
+41 (0)61 467 85 44
support@swisshealthweb.ch
www.swisshealthweb.ch
Published on 20.04.2020
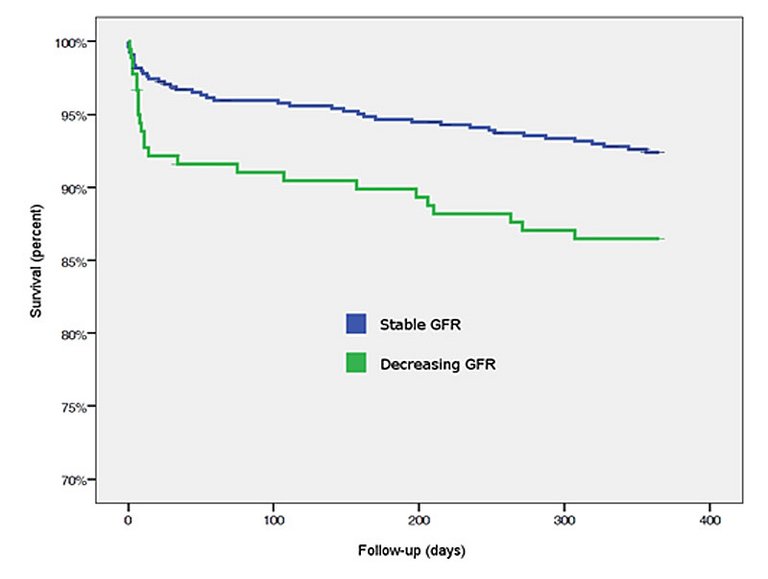
Decreasing glomerular filtration rate within 24 hours after admission for an acute coronary syndrome is significantly associated with cardiovascular and bleeding events at 30 days and 1 year.
| Frequency (n = 724) | Percent (%) | ||
|---|---|---|---|
| GFR class | Stable | 545 | 69.3 |
| Decreasing | 179 | 22.7 | |
| Creatinine change | <0.3 mg/dl | 663 | 91.6 |
| ≥0.3 mg/dl | 61 | 8.4 | |
| Stable GFR (n = 545) | Decreasing GFR (n = 179) | p-value | |
|---|---|---|---|
| Female, n (%) | 92 (16.9) | 56 (31.3) | <0.00 |
| Age (years), mean | 61.903 | 69.181 | <0.00 |
| BMI (kg/m2), mean | 27.310 | 25.725 | <0.00 |
| Smoking history (current), n (%) | 239 (43.9) | 55 (30.7) | <0.00 |
| Smoking history (former), n (%) | 167 (30.6) | 47 (26.3) | <0.00 |
| Systolic BP (mm Hg), mean (SD) | 131.806 (24.83) | 132.326 (23.18) | 0.80 |
| Diastolic BP (mm Hg), mean (SD) | 78.596 (15.29) | 77.129 (14.97) | 0.27 |
| LVEF before procedure, % | 52.18 | 53.30 | 0.30 |
| Stable GFR (n = 545) | Decreasing GFR (n = 179) | p-value | |
|---|---|---|---|
| n (%) | n (%) | ||
| Coronary artery disease | 139 (71.6) | 45 (69.3) | 0.26 |
| Peripheral artery disease | 24 (4.4) | 19 (10.6) | 0.01 |
| Hypertension | 325 (59.6) | 115 (64.2) | 0.29 |
| Cholesterolaemia | 377 (69.3) | 117 (65.7) | 0.40 |
| Valvular heart disease | 5 (0.9) | 2 (1.1) | 0.69 |
| Congestive heart failure | 9 (1.7) | 5 (2.8) | 0.35 |
| Myocardial infarction history | 74 (13.6) | 16 (8.9) | 0.12 |
| PCI history | 87 (16) | 30 (16.8) | 0.82 |
| CABG history | 28 (5.1) | 8 (4.5) | 0.84 |
| Dialysis | 3 (0.6) | 0 (0.0) | 1.00 |
| Diabetes | 101 (18.5) | 33 (18.4) | 1.00 |
| ‒ Insulin-dependent diabetes | 23 (4.2) | 13 (7.3) | 0.11 |
| Stroke history | 16 (2.9) | 5 (2.8) | 1.00 |
| TIA history | 6 (1.1) | 3 (1.7) | 0.70 |
| Malignancy | 48 (8.8) | 14 (7.8) | 0.76 |
| Lung disease | 26 (4.8) | 7 (3.9) | 0.84 |
| Inflammatory disease | 16 (2.9) | 8 (4.5) | 0.34 |
| Gastrointestinal bleeding | 10 (1.8) | 6 (3.4) | 0.24 |
| Liver disease | 5 (0.9) | 1 (0.6) | 1.00 |
| Stable GFR (n = 545) | Decreasing GFR (n = 179) | p-value | ||
|---|---|---|---|---|
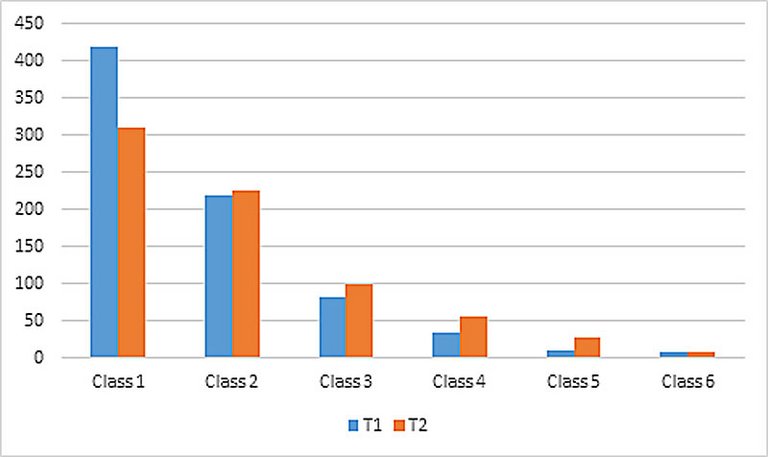
| Creatinine (mg/dl) | T1 | 86.292 (SD 57.35) | 85.497 (SD 28.59) | 0.86 |
| T2 | 93.174 (SD 60.82) | 116.067 (SD 59.84) | 0.00 | |
| HsTnT (μg/l) | T1 | 0.218 (SD 4.36) | 0.305 (SD 0.84) | 0.62 |
| T2 | 1.970 (SD 5.53) | 2.660 (SD 7.05) | 0.10 | |
| CK (U/l) | T1 | 272.000 (IR 512.0) | 239.000 (IR 437.5) | 0.40 |
| T2 | 823.000 (SD 1771.25) | 968.000 (SD 2027.75) | 0.06 | |
| CK-MB (U/l) | T1 | 34.000 (IR 57.5) | 32.000 (IR 71.0) | 0.91 |
| T2 | 104.000 (IR 196.5) | 105.000 (IR 246.0) | 0.11 | |
| Haemoglobin (g/dl) | T1 | 13.950 (SD 1.9) | 13.369 (SD 1.7) | 0.00 |
| Total patients (n = 724) | Stable GFR (n = 545) | Decreasing GFR (n = 179) | p-value | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
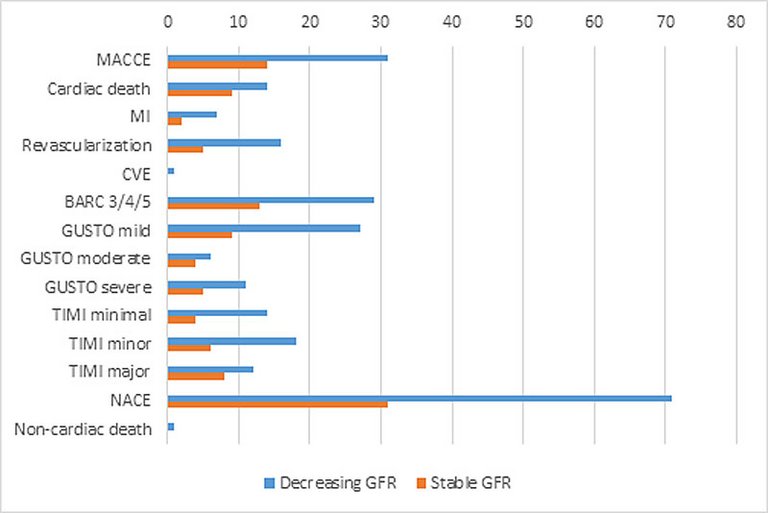
| MACCE | 31 (4.3) | 17 (3.1) | 14 (7.8) | 0.01 |
| ‒ Cardiac death | 14 (1.9) | 5 (0.9) | 9 (5) | 0.00 |
| ‒ MI | 7 (1) | 5 (0.9) | 2 (1.1) | 0.69 |
| ‒ Revascularisation | 16 (2.2) | 11 (2.0) | 5 (2.8) | 0.56 |
| ‒ CVE | 1 (0.1) | 1 (0.2) | 0 (0) | 1.00 |
| BARC 3/4/5 | 29 (4) | 16 (2.9) | 13 (7.3) | 0.02 |
| GUSTO | ||||
| ‒ Mild | 27 (3.7) | 18 (3.3) | 9 (5.0) | 0.36 |
| ‒ Moderate | 6 (0.8) | 2 (0.4) | 4 (2.2) | 0.04 |
| ‒ Severe | 11 (1.5) | 6 (1.1) | 5 (2.8) | 0.15 |
| TIMI | ||||
| ‒ Minimal | 14 (1.9) | 10 (1.8) | 4 (2.2) | 0.76 |
| ‒ Minor | 18 (2.5) | 12 (2.2) | 6 (3.4) | 0.41 |
| ‒ Major | 12 (1.7) | 4 (0.7) | 8 (4.5) | 0.00 |
| NACE | 71 (9.8) | 40 (7.3) | 31 (17.3) | <0.00 |
| Noncardiac death | 1 (0.1) | 1 (0.2) | 0 (0) | 1.00 |
| Total patients (n = 724) | Stable GFR (n = 545) | Decreasing GFR (n = 179) | p-value | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
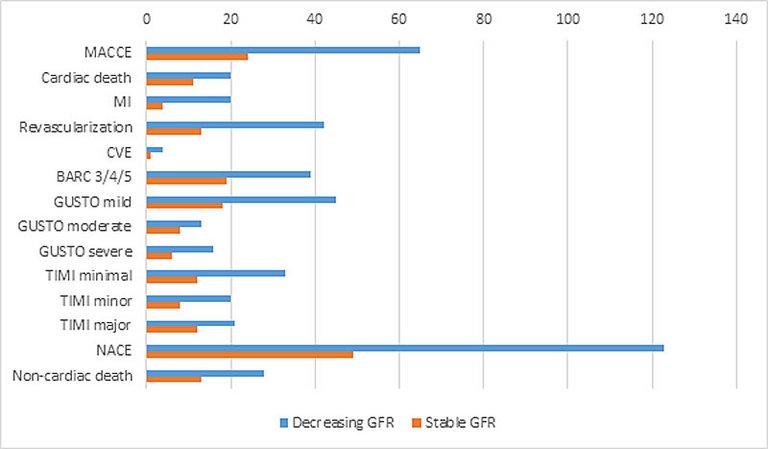
| MACCE | 65 (9) | 41 (7.5) | 24 (13.4) | 0.02 |
| ‒ Cardiac death | 20 (2.8) | 9 (1.7) | 11 (6.1) | 0.00 |
| ‒ MI | 20 (2.8) | 16 (2.9) | 4 (2.2) | 0.76 |
| ‒ Revascularisation | 42 (5.8) | 29 (5.3) | 13 (7.3) | 0.36 |
| ‒ CVE | 4 (0.6) | 3 (0.6) | 1 (0.6) | 1.00 |
| BARC 3/4/5 | 39 (5.4) | 20 (3.7) | 19 (10.6) | 0.00 |
| GUSTO | ||||
| ‒ Mild | 45 (6.2) | 27 (5) | 18 (10.1) | 0.02 |
| ‒ Moderate | 13 (1.8) | 5 (0.9) | 8 (4.5) | 0.01 |
| ‒ Severe | 16 (2.2) | 10 (1.8) | 6 (3.4) | 0.24 |
| TIMI | ||||
| ‒ Minimal | 33 (4.6) | 21 (3.9) | 12 (6.7) | 0.15 |
| ‒ Minor | 20 (2.8) | 12 (2.2) | 8 (4.5) | 0.12 |
| ‒ Major | 21 (2.9) | 9 (1.7) | 12 (6.7) | 0.00 |
| NACE | 123 (17) | 74 (13.6) | 49 (27.4) | <0.00 |
| Noncardiac death | 28 (3.9) | 15 (2.8) | 13 (7.3) | 0.01 |
Published under the copyright license.
"Attribution - Non-Commercial - NoDerivatives 4.0"
No commercial reuse without permission.
See: emh.ch/en/emh/rights-and-licences/